Material on this page is copyright © 2016 John W.F. Waldron
This section is about the different ways in which rock materials respond to stress. Elastic responses and brittle fracture were covered in an earlier section.
For each material under a particular range of conditions, there is a relationship between stress and strain, which is called the constitutive law or flow law. In elastic deformation, strain and stress are proportional, a relationship expressed in Hooke's Law. In studying ductile deformation, the flow law is typically a relationship between stress and strain rate; strain continues to be acquired while stress is applied, but when the stress is removed, the strain is retained. We say that the deformation is non-recoverable.
At high mean stress (pressure) there is a transition from brittle deformation to permanent (non-recoverable) deformation that is not associated with a loss of cohesion, termed continuous deformation, flow, ductile flow or creep.
This transition can be seen in stress tests at high confining pressure and high temperature.
In creep, it is the strain rate, not the finite strain, that is a function of the applied stress. Strain rate is represented by de/dt or by
It's possible to calculate bond strengths in crystals from chemical data. In most cases, the strengths indicate that it's impossible, with geologically reasonable stresses, to simultaneously break all the bonds in a crystal, so as to (for example) shear the crystal.
Nevertheless, we see clear evidence for distortion of crystals in deformed rocks.
It turns out that crystal defects - departures from the ideal lattice structure - are critically important in allowing crystals to deform.
In general, defects affect either points, lines, or planes within the crystal lattice.
Point defects include:
Point defects can move through crystals by diffusion (random motion of atoms). Diffusion takes place in all crystalline materials, but the rate of diffusion increases with temperature. Vacancies are particularly important in allowing diffusion to take place
Crystal changes shape by migration of point defects - particularly vacancies - through the crystal.
Crystal changes shape by migration of atoms along grain boundaries. This works better at small grain sizes because the area of grain boundary is large.
Rate depends on amount, pressure, and chemistry of fluid present. Leads to development of stylolites and pressure solution cleavage
Grains slide past each other; corners and other irregularities that would normally prevent grain-boundary-sliding can be diffused out of the way leaving behind a fine-grained aggregate that does not show strong CPO.
Crystals undergoing deformation by solid-state diffusion typically show a strain rate that is proportional to the differential stress.
This type of stress-strain relationship is known as viscous or Newtonian.
The equation for viscous flow is
![]()
or ![]()
where η is the viscosity and σd is the differential stress σ1 - σ3
Strain rates for superplasticity are different. Superplastic typically follows a power law relationship in which strain is proportional to stress raised to some power n typically between 1.4 and 2
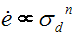
Line defects are also known as dislocations.
The nature of the defect is defined by the Burger's vector b.
To define the Burger's vector, describe a closed loop in an undeformed section of crystal. Then follow a similar loop around the dislocation; the gap between the start and end point is the Burger's vector b.
An edge dislocation has b perpendicular to the dislocation line. It marks the edge of an extra half-plane of atoms.
Large numbers of dislocations produce a curved crystal lattice
This shows up in thin section as undulatory or undulose extinction
A screw dislocation has b parallel to the dislocation line. Atoms are connected in a helix ("spiral staircase") around the screw dislocation.
In practice, a given dislocation can change orientation from parallel to perpendicular to b, and all orientations in between.
Dislocation glide is the fundamental process of deformation at high strain rates, by which mylonites flow
The constitutive law for dislocation glide is typically exponential creep.
![]()
Dislocation glide leads to huge densities of dislocations within crystals. Dislocation densities are often stated in units of cm-2. Highly strained quartz may have a dislocation density of 1011 cm-2. (That means every cubic centimetre of quartz contains a mind-boggling hundred thousand kilometres of dislocations!) Eventually this leads to strain hardening because the dislocations get tangled - they need good, intact crystal to migrate, and so are held up by other dislocations
However, at slower strain rates, there are other processes involving diffusion that can assist with dislocation motion.
Deformation involving a combination of diffusion with dislocation glide are known as dislocation creep. In metallurgy, diffusion-assisted reorganization of dislocations is known as recovery.
Several processes are involved in dislocation creep and recovery:
Dislocation creep typically follows a power law relationship in which strain is proportional to stress raised to some power between 1 and 5, typically around 3
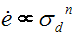
Notice that the crystal lattice is distorted in the vicinity of a dislocation wherever one occurs. This results in availability of energy to break bonds, and thus there is a tendency for a crystal containing a lot of dislocations to rebuild itself into numerous small crystals. This is the process of dynamic recrystallization that produces mylonite.
Dynamic recrystallization produces a strong crystal preferred orientation (CPO) or lattice preferred orientation (LPO).
Planar defects can also play a role in deformation
Typically, across a twin plane, the structure of a crystal is reflected. (Rotations are also possible). Calcite and plagioclase feldspar are two minerals that typically show abundant twin planes.
Strain twinning (twin gliding) is a major mechanism of deformation in carbonates
Twin gliding seems to follow a flow law that is exponential, similar to dislocation glide.
![]()
Grain boundaries can be thought of as major planar defects, and are associated with extra energy (surface energy) that can be released by the transfer of atoms across grain boundaries
For most of these types of deformation it's now possible to determine a theoretical relationship between stress and strain, based on knowledge of the kinetic behaviour of atoms at various temperatures, and on the bond strengths for different types of bonds (from chemistry).
Hence we can determine which mechanism gives the fastest strain rate at a given set of conditions (temperature and differential stress).
This leads to the idea of a deformation map - typically a plot with temperature and differential stress on the axes, divided into areas according to which mechanism is fastest.
These maps show the fastest deformation mechanism under given conditions of T and differential stress. Contours are drawn within the diagrams showing the strain rate predicted under those given conditions. (Note: the lines are numbered with the log of the strain rate: where it says '-9' this means 10-9).
In general, only the highest strain rates can be experimentally verified: the lower strain-rate parts of the diagram are predicted based on chemically determined diffusion constants and bond strengths.
We can use these diagrams to make predictions about the strength of the lithosphere at various depths, by assuming a geothermal gradient (rate of temperature increase with depth).
In order to do this we have to bring brittle deformation into the picture. Brittle deformation is strongly dependent on pressure. The Mohr-Coulomb failure relationship or 'Byerlee's Law' allows us to predict the increasing differential stress required for fracture with increasing pressure. Hence the strength depth relationship shows an initial more or less linear increase of strength with depth.
(The relationship is a little different depending on whether the lithosphere is being extended or shortened. In general if the maximum compressive stress is vertical, a rather lower differential stress is required than if the maximum compressive stress is horizontal. This follows from the Mohr construction for brittle failure.)
At some depth, the brittle deformation curve intersects the strength-depth curve for one of the ductile mechanisms. This point marks the brittle-ductile transistion. Below this point, creep requires less differential stress than fracture, so rocks flow.
If we are going to make deductions about the strength of the lithosphere we need to assume some compositions: in the diagrams we often assume that quartz is the strength controlling-mineral in the crust, and that olivine controls strength in the mantle. For an average continental geothermal gradient this predicts a strong mid-upper crust, a weak lower crust, and a strong upper mantle.
We can use somewhat more sophisticated models. E.g. a crust that has a wet-quartz-dominated 'granitic' upper layer, a dry-quartz-dominated mid crust, a feldspar-dominated 'granulite' lower crustal layer, and an olivine-dominated peridotite lithospheric mantle.